1. Introduction
Electroplating is defined as an electrochemical process in which a thin layer of metal is deposited on a substrate using an electrolyte solution, an anode, and a cathode.
This process not only enhances the performance and durability of the base material but also improves its visual appeal and functionality.
Historically, electroplating evolved from early 19th-century experiments into the sophisticated,
automated systems used today, largely driven by advancements in materials science and environmental regulations.
Industries such as automotive, aerospace, electronics, jewelry, and medical devices rely on electroplating to achieve consistent and high-quality finishes.
In this article, we aim to analyze electroplating through multiple lenses—exploring its scientific foundations, process optimization, design considerations, economic impacts, environmental challenges, and emerging trends.
This multi-dimensional approach ensures a comprehensive understanding of the technology that underpins modern surface finishing.
2. Fundamentals of Electroplating
What is Electroplating?
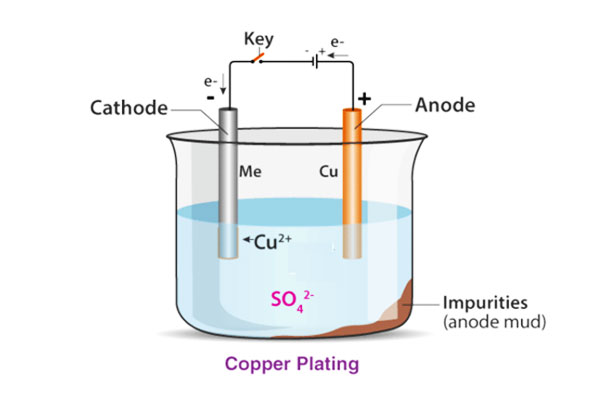
Electroplating involves immersing the substrate (cathode) and a metal source (anode) in an electrolyte solution and then applying an electrical current.
As electrons flow, metal ions from the anode are reduced and deposited onto the substrate, forming a thin, uniform coating.
This process improves properties such as corrosion resistance and durability while also enhancing the component’s appearance.
Electrochemical Principles
At the heart of electroplating lie redox reactions. The metal at the anode oxidizes into ions, which migrate through the electrolyte and are reduced on the cathode.
For instance, during nickel electroplating, nickel atoms from the anode oxidize, dissolve into the solution as ions, and are subsequently deposited as a solid layer onto the workpiece.
Key process parameters—such as current density, voltage, electrolyte composition, and temperature—directly influence deposition quality.
Notably, higher current densities can accelerate deposition rates but may also cause rougher finishes if not carefully controlled.
3. Scientific and Theoretical Foundations
Material Science Perspective
The quality of an electroplated coating depends significantly on the material science behind it.
Atomic bonding and nucleation mechanisms determine how well the deposited metal adheres to the substrate.
For example, the formation of initial nucleation sites and subsequent growth can lead to a uniform and dense coating, which is critical for corrosion resistance.
Studies have shown that optimizing electrolyte composition and surface activation can improve adhesion by up to 20%.
Theoretical Models
Electrode kinetics, mass transport phenomena, and thermodynamic factors are essential in predicting plating outcomes.
Diffusion, migration, and convection all affect how metal ions move within the electrolyte.
Additionally, the overpotential required for deposition and equilibrium conditions dictate the quality of the deposit.
Mathematical models and simulations are increasingly used to predict these interactions, leading to more controlled and efficient processes.
Empirical Data and Validation
Empirical data validate these theoretical models.
For instance, nickel plating typically deposits at rates ranging from 1 to 3 µm per minute, while decorative chrome coatings might target thicknesses between 5 and 10 µm.
Such data are critical for process optimization and quality control, as even a 10% variation in current density can result in noticeable differences in coating thickness and performance.
4. Types of Electroplating
Electroplating encompasses a variety of techniques designed to deposit metal layers onto substrates, each tailored for specific applications and performance requirements.
By leveraging different methods, manufacturers can achieve coatings with distinct properties, ranging from decorative finishes to high-performance protective layers.
In this section, we examine the primary types of electroplating, discussing standard methods, specialized techniques, and emerging alternatives.
4.1 Standard Electroplating Techniques
Standard electroplating methods form the backbone of industrial applications.
These conventional processes reliably deposit metal coatings such as nickel, chromium, copper, and gold onto substrates.
Nickel and Chromium Plating
- Nickel Plating:
Nickel plating is widely used for its excellent corrosion resistance and hardness.
In automotive and industrial applications, nickel coatings typically achieve thicknesses between 5 and 15 microns.
For instance, automotive components like engine parts and chassis often employ nickel plating to extend their service life under harsh operating conditions.Nickel Plating - Chromium Plating:
Chrome plating offers a high-gloss, mirror-like finish, and outstanding wear resistance. It finds extensive use in decorative applications as well as in heavy-duty industries.
Decorative chrome coatings usually range from 5 to 10 microns in thickness, providing both a refined appearance and robust surface protection.
Copper and Gold Plating
- Copper Plating:
Copper plating improves electrical conductivity and thermal performance, making it a staple in electronic applications.
Typical deposition rates range from 2 to 4 microns per minute, ensuring consistent and reliable coatings on circuit boards and connectors. - Gold Plating:
Known for its superior conductivity and resistance to tarnish, gold plating is common in high-end electronics and jewelry.
Although gold plating is usually thinner—often less than 5 microns—it adds significant value by enhancing both performance and appearance.Gold Plating
4.2 Specialized Electroplating Techniques
Beyond standard methods, specialized techniques address unique industry challenges by offering greater control and tailored coating properties.
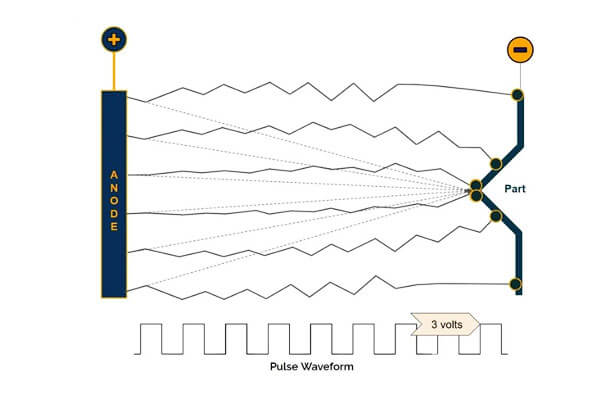
Pulse Plating
Pulse plating employs intermittent bursts of electrical current instead of a constant direct current. This method offers several key advantages:
- Enhanced Control:
Pulse plating allows precise control over deposition kinetics, reducing internal stresses and refining the microstructure.
Research indicates that pulse plating can reduce surface roughness by up to 30% compared to conventional methods. - Improved Coating Quality:
It results in a finer grain structure, which enhances the coating’s adhesion and uniformity—vital for high-precision applications in aerospace and advanced tooling.
Brush Plating
Brush plating is a localized plating technique, ideal for repair and touch-up applications:
- Targeted Application:
Using a brush-like tool, operators can selectively deposit metal coatings on damaged areas without immersing the entire part in an electrolyte bath. - Cost-Effective Repair:
Brush plating proves particularly useful in maintenance operations, reducing downtime and saving costs by avoiding complete re-plating of parts.
Micro-Plating
Micro-plating techniques enable the deposition of ultra-thin metal layers—often in the sub-micron range—essential for precision electronics and semiconductor devices:
- Precision Control:
Micro-plating achieves uniform coatings with thicknesses typically below 1 micron, crucial for high-density circuit boards and microelectromechanical systems (MEMS). - Minimal Material Addition:
This technique ensures that the added weight remains negligible while providing enhanced functionality such as improved conductivity or corrosion resistance.
Electroless Plating (Chemical Plating)
Electroless plating, although not strictly an electroplating method, shares similarities by depositing metal without an external electrical current:
- Uniformity on Complex Surfaces:
It yields uniform coatings even on irregular or porous surfaces, making it ideal for non-conductive materials that require a conductive layer. - Wide Industrial Use:
Electroless nickel plating, for example, is prevalent in the aerospace and automotive industries due to its excellent corrosion resistance and ability to form a consistent, dense layer.
4.3 Emerging and Hybrid Techniques
Advancements in technology have led to the development of hybrid techniques that combine the strengths of various methods to meet ever-evolving industrial demands.
Hybrid Electroplating Techniques
- Combination Processes:
Hybrid techniques integrate electroplating with other surface treatment methods such as thermal spraying or anodizing.
These combinations aim to enhance overall performance by merging the benefits of each process.
For example, an initial electroplated layer may be further treated with thermal spraying to achieve a thicker, more robust coating. - Process Customization:
Engineers are increasingly using simulation tools and machine learning to customize hybrid processes.
These digital innovations optimize parameters in real-time, reducing material waste and ensuring consistent quality.
4.4 Comparative Analysis and Selection Criteria
Choosing the right electroplating method depends on a host of factors. Consider the following criteria:
Substrate Material:
The choice of plating technique often depends on the substrate.
For instance, delicate electronic components benefit from micro-plating, while larger automotive parts are better served by standard nickel or chrome plating.
Desired Coating Properties:
Factors such as thickness, uniformity, adhesion, and mechanical strength influence the selection process.
Pulse plating, for example, excels in applications where reducing internal stress is critical.
Cost and Efficiency:
The economic implications, including equipment investment, operational costs, and throughput, also play a significant role.
While specialized techniques like micro-plating offer superior precision, they may incur higher costs compared to conventional methods.
Environmental and Regulatory Considerations:
Sustainable practices and compliance with environmental regulations may favor one technique over another.
Electroless plating and advanced pulse plating methods, for example, can reduce chemical waste and energy consumption.
5. Common Process of Electroplating
The electroplating process is a meticulously controlled sequence of steps that transforms a bare substrate into a coated component with enhanced performance and aesthetic qualities.
This process not only improves properties such as corrosion resistance and conductivity but also plays a critical role in quality control.
Below, we detail each phase of the electroplating process, supported by data and real-world examples, to illustrate how each step contributes to a high-quality finish.
Pre-treatment and Surface Preparation
A successful electroplating operation starts with thorough surface preparation.
This stage is vital because even the most advanced plating technology cannot overcome the adhesion problems caused by contaminants or surface irregularities.
- Cleaning and Degreasing:
The substrate must be cleaned to remove oils, dirt, and other impurities.
Common cleaning agents include alkaline solutions and solvents, which can reduce surface contamination by over 95%.
For example, automated cleaning systems in the automotive industry ensure that each part meets stringent cleanliness standards before plating. - Etching and Activation:
Acid or alkaline etching removes any residual oxides and roughens the surface, creating microscopic features that enhance mechanical bonding.
Proper etching can improve coating adhesion by 15–20%. This step is particularly important for metals like stainless steel, where passive oxide layers can inhibit deposition. - Rinsing:
Rinsing with deionized water ensures that all chemical residues are removed. Consistent rinsing protocols help maintain uniform surface conditions, minimizing defects in the final coating.
Plating Operation
Once the substrate is properly prepared, the plating operation begins.

This phase involves immersing the cleaned workpiece into an electrolyte bath, where a controlled electrical current facilitates the deposition of metal ions onto the surface.
- Electrolyte Bath Composition:
The plating solution contains dissolved metal salts and additives that control the deposition process.
For instance, a nickel plating bath might contain nickel sulfate, nickel chloride, and boric acid.
Maintaining precise chemical concentrations ensures consistent deposition rates, which typically range from 1 to 3 µm per minute for industrial applications. - Current and Voltage Control:
Applying a direct current drives the metal ions toward the workpiece (cathode).
The current density is critical: too high, and the coating may become rough and porous; too low, and the deposition rate will be inefficient.
Modern systems use computerized controls to maintain optimal conditions and adjust parameters in real-time. - Deposition Phase:
During this phase, metal ions are reduced at the cathode, forming a coherent and adherent metal layer.
For example, electroplated chrome coatings often target a thickness of 5–10 µm, providing both decorative appeal and enhanced wear resistance.
Post-Treatment Processes
After deposition, post-treatment processes refine the electroplated coating, ensuring durability and performance.
- Rinsing and Drying:
Following electroplating, the workpiece is thoroughly rinsed to remove any residual electrolytes.
It is then dried using forced-air or infrared systems, which are designed to avoid water spots or uneven drying. - Sealing and Passivation:
Some applications require an additional sealing step to further enhance corrosion resistance.
For instance, after nickel plating, a passivation treatment can improve the coating’s resistance to environmental degradation, extending the component’s service life by up to 25%. - Inspection and Quality Control:
Rigorous quality control measures, including thickness measurements and adhesion tests, ensure that the coating meets specifications.
Automated optical and mechanical inspection systems are widely used, reducing the rate of defective parts to below 2%.
Quality Control and Process Optimization
Maintaining consistent quality throughout the electroplating process is essential.
Integrated monitoring systems track key parameters such as current density, bath temperature, and chemical composition.
This data-driven approach allows manufacturers to adjust the process in real-time, ensuring uniformity and reducing waste.
- Real-Time Monitoring:
Sensors continuously measure conditions in the plating bath, alerting operators to any deviations. Such systems can improve process efficiency by up to 20%. - Statistical Process Control (SPC):
Employing SPC methods helps identify trends and maintain quality over large production runs.
Companies report significant reductions in scrap rates and rework when using these advanced quality control strategies.
6. Advantages and Disadvantages of Electroplating
Advantages
- Enhanced Functional Properties:
Electroplating significantly improves corrosion resistance, wear resistance, electrical conductivity, and thermal performance. - Aesthetic Versatility:
Achieve high-gloss, uniform finishes in a wide range of colors (gold, silver, chrome) for decorative and functional applications. - Cost-Effective for High-Volume Production:
Once optimized, electroplating processes reduce material waste and extend component lifespans, delivering long-term savings. - Customization:
Ability to control coating thickness and tailor properties to meet specific application demands.
Disadvantages
- Environmental and Safety Concerns:
The use of hazardous chemicals requires stringent waste management and safety protocols. - High Initial Capital Investment:
Setting up an advanced electroplating facility demands significant investment in equipment and infrastructure. - Process Sensitivity:
Variability in electrolyte composition, temperature, and current can lead to inconsistent coatings if not properly controlled. - Material Limitations:
Some substrates require specialized pre-treatment to ensure proper adhesion, adding complexity to the process.
7. Applications of Electroplating: Industry-Specific Uses and Benefits
Electroplating plays a crucial role in various industries, providing enhanced surface properties, corrosion protection, aesthetic appeal, and functional improvements.
Below is a detailed exploration of its applications across major sectors.
Automotive Industry
Electroplating is widely used in the manufacturing of vehicle components to improve durability, wear resistance, and appearance.
Key Applications:
- Chrome Plating: Used on bumpers, grilles, and trim for aesthetic appeal and corrosion resistance.
- Nickel Plating: Applied to engine components, pistons, and gears for wear resistance and longevity.
- Zinc and Zinc-Nickel Plating: Protects underbody components, fasteners, and chassis parts from corrosion and environmental damage.
- Copper-Nickel-Chrome Plating: Used in exhaust systems for heat and oxidation resistance.
Aerospace Industry
Aircraft components require high-performance coatings to withstand extreme temperature variations, mechanical stress, and corrosive environments.
Key Applications:
- Cadmium Plating: Used on landing gear, fasteners, and critical structural components for corrosion resistance.
- Hard Chrome Plating: Applied to hydraulic cylinders, aircraft bearings, and turbine shafts for high wear resistance and durability.
- Nickel Plating: Provides oxidation resistance and thermal stability in jet engine components.
Electronics and Semiconductor Industry
Electroplating is essential for manufacturing electronic circuits, connectors, and micro-components, ensuring high electrical conductivity and durability.
Key Applications:
- Gold and Silver Plating: Used for high-conductivity contacts in circuit boards, connectors, and semiconductor chips.
- Copper Plating: Applied in printed circuit boards (PCBs) to enhance electrical pathways and improve thermal dissipation.
- Nickel and Tin Plating: Used in electronic connectors to prevent oxidation and ensure long-term reliability.
Medical and Biomedical Applications
Medical devices require biocompatible and durable coatings to prevent wear, corrosion, and bacterial contamination.
Key Applications:
- Gold and Silver Plating: Used in electrodes, pacemakers, and surgical instruments for biocompatibility and electrical conductivity.
- Nickel-Titanium (NiTi) Coatings: Applied on orthopedic implants and dental tools for enhanced mechanical strength.
- Chrome and Nickel Plating: Used on surgical tools and prosthetics to ensure sterility and wear resistance.
Jewelry and Luxury Goods
Electroplating enhances the appearance, durability, and value of precious metal products.
Key Applications:
- Gold and Rhodium Plating: Used for tarnish resistance and luster in rings, watches, and luxury accessories.
- Silver and Platinum Plating: Applied to enhance scratch resistance and brilliance in high-end jewelry.
Industrial Machinery and Tools
Industrial tools and equipment undergo intense mechanical stress, requiring protective coatings to extend service life.
Key Applications:
- Hard Chrome Plating: Used in dies, molds, and cutting tools for abrasion resistance and hardness.
- Nickel and Cobalt Plating: Enhances corrosion resistance and heat tolerance in heavy-duty industrial parts.
8. Design Considerations for Electroplating
Substrate Compatibility
- Evaluate the type and condition of the substrate, ensuring it can withstand the electroplating process.
- Consider pre-treatment requirements to maximize adhesion and uniformity.
Coating Specifications
- Determine the optimal thickness, finish, and adhesion needed based on performance and aesthetic requirements.
- Use design simulations and empirical data to guide process parameters.
Process Integration
- Integrate electroplating seamlessly into existing manufacturing workflows.
- Decide between batch and continuous processes based on production volume and cost-effectiveness.
Environmental and Safety Compliance
- Ensure that the electroplating process meets international environmental regulations (e.g., EPA, REACH).
- Implement proper waste management and safety protocols to protect workers and the environment.
Cost-Benefit Trade-Offs
- Assess the economic impact of electroplating relative to other finishing methods.
- Consider long-term benefits such as extended product life and reduced maintenance costs against initial capital expenditures.
9. Comparison of Electroplating with Other Surface Treatments
Below is a direct side-by-side comparison of electroplating with other common surface treatment methods, summarizing key performance indicators.
| Feature | Electroplating | Anodizing | Powder Coating | PVD/CVD Coating | Galvanizing | Thermal Spraying |
|---|---|---|---|---|---|---|
| Process | Electrochemical metal deposition | Electrochemical oxidation | Electrostatic dry powder + curing | Vapor-phase coating in a vacuum | Molten zinc bath | Spraying molten/semi-molten material |
| Typical Materials | Various metals | Aluminum, titanium | Metals, some plastics | Metals, ceramics, plastics | Steel, iron | Metals, ceramics, polymers |
| Corrosion Resistance | High (nickel, chrome, zinc plating) | High (oxide layer) | High (epoxy coatings) | Excellent | Very high | Very high |
| Wear Resistance | Good, depends on metal | Excellent | Good, chip-resistant | Superior, extreme hardness | Moderate | Excellent, used in extreme environments |
| Aesthetic Appeal | Bright, decorative metallic finishes | Matte, can be dyed | Wide range of colors/textures, no metallic sheen | Metallic and colored finishes | Dull, rough industrial finish | Rough, industrial appearance |
| Durability | Moderate to high | High | Very high, resists cracking | Extremely high | High for outdoor exposure | Extremely high, aerospace-grade |
| Cost | Moderate to high | Low to moderate | Low per unit for bulk | High, due to vacuum processing | Low, cost-effective for steel | High, requires specialized equipment |
| Environmental Impact | Chemical waste, hazardous materials | Eco-friendly, minimal waste | Eco-friendly, no VOCs | Minimal waste, green technology | Produces zinc waste but recyclable | Some materials may be hazardous |
| Applications | Jewelry, electronics, automotive, aerospace | Aerospace, consumer goods, construction | Consumer goods, industrial parts, architecture | Cutting tools, aerospace, medical implants | Structural steel, bridges, automotive underbodies | Aerospace, turbines, biomedical implants |
10. Future Trends and Innovations
Technological Advancements
- Advanced Plating Techniques:
Explore pulse plating, brush plating, and micro-plating, which offer enhanced control over deposition and reduced internal stresses. - Digital Integration:
Analyze how IoT, AI, and real-time monitoring systems optimize process control and predictive maintenance. - Nanotechnology:
Examine how nano-scale additives improve coating performance, durability, and electrical properties.
Market Dynamics and Global Outlook
- Present forecasts show market growth, with projections indicating a CAGR of 5-7% over the next decade.
- Discuss regional trends, highlighting differences between markets in North America, Europe, and Asia-Pacific.
- Identify strategic opportunities for companies investing in eco-friendly and advanced electroplating technologies.
Sustainability and Regulatory Developments
- Investigate emerging eco-friendly practices, including the use of bio-based electrolytes and low-emission processes.
- Forecast how evolving regulations will drive further innovation and adoption of green electroplating methods.
11. Conclusion
In summary, electroplating is a multifaceted process that plays a vital role in enhancing the performance, durability, and appearance of materials across a diverse range of industries.
Through a thorough understanding of its scientific principles, process optimization, and design considerations, manufacturers can leverage electroplating to achieve superior product quality and sustainability.
Advancements in digital integration, nanotechnology, and eco-friendly practices promise to further enhance the efficiency and environmental compatibility of electroplating.
As global markets continue to evolve, the ability to innovate and adapt in electroplating will remain crucial for maintaining competitiveness and driving industrial progress.
If you’re looking for high-quality surface treatment services, choosing LangHe is the perfect decision for your manufacturing needs.